
The pharmaceutical and biotechnology sector, which is subject to colossal financial stakes, is constantly seeking the right balance between long, costly and economically risky R&D activities and mass production activities, which require high capacity investments and whose return on investment is consolidated by the speed of marketing and the life span of the products manufactured.
For several years, the pharmaceutical and biotechnology sector has had to adapt to certain structural changes in its market:
As a result, the sector is in a constant evolving, both in terms of the products, production methods and equipment required and the diversity of the players and technologies involved. These developments must be carried out with the absolute guarantee of the quality control of the products supplied, the respect of the constantly evolving regulatory constraints and the simplification of the compliance processes.
The scope of the cosmetics industry is being reconfigured with the integration of two strong trends: organic cosmetics and personalised cosmetics. Sales of organic and natural products have increased significantly and the adoption of an international standard on organic cosmetics has made the market more transparent. In this market, manufacturers also offer products adapted to each skin, to the aggressiveness of the environment in which one evolves, to one's state of fitness, fatigue, etc.
With its expertise in contamination control and Controlled Atmosphere Zone (CAZ) engineering, ATRIX offers project owners cutting-edge solutions for the engineering controlled environments (laboratories, clean rooms, sterile rooms), integrating the notion of energy savings and meeting the sector's requirements in terms of standards and procedures.
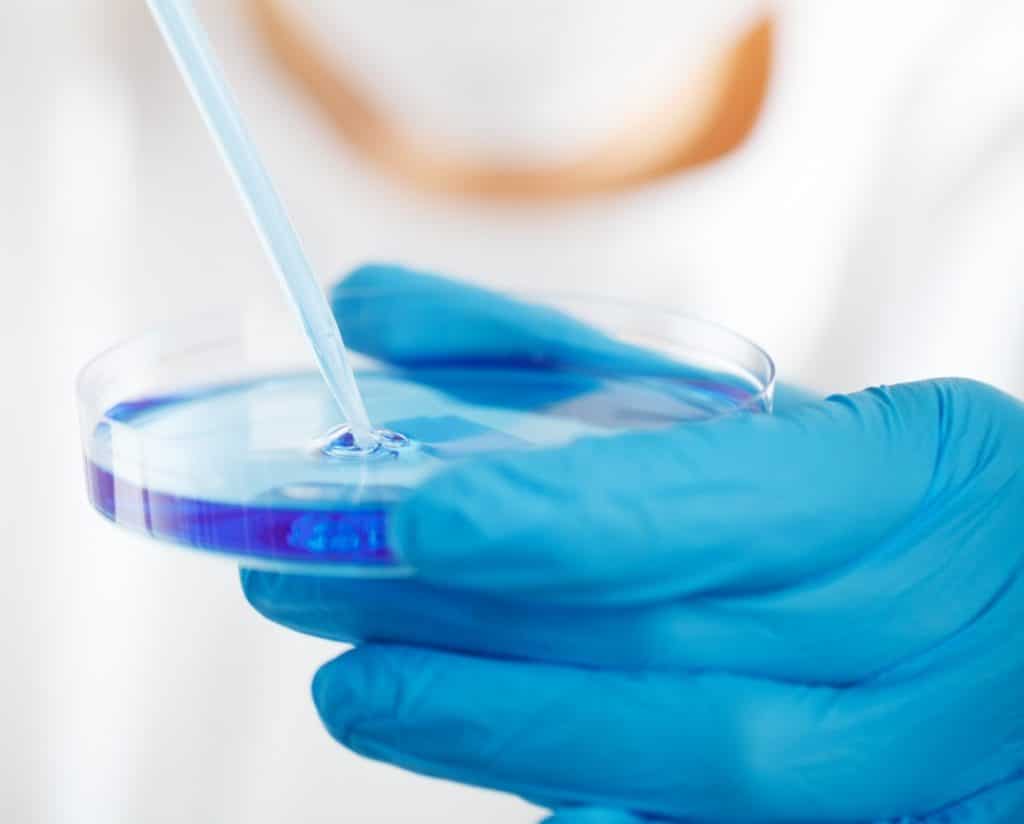
In addition to its technical skills, in particular with regard to clean utilities (EPU, EPPI, VP, ACP, etc.), the Group provides its clients with its knowledge of pharmaceutical processes (dry forms, liquid forms, sterile injectable products, vaccines, etc.) and environments associated with the control of Good Manufacturing Practices (GMP) and compliance with the requirements of the European (EP) and American (USP, FDA) pharmacopoeias.
This expertise, together with the group's architects, enables ATRIX to offer a real global approach to the projects entrusted to us by our clients, favouring the perfect handling of process constraints in ergonomic buildings that are ideally integrated into their host sites.
ARCHITECTURE AND PROJECTS
With the support of all the group’s expertise, our architects and project engineers manage our project management, project management assistance or engineering operations integrated into design and construction contracts. ATRIX is thus able to commit to results objectives in terms of performance, costs and schedules.
Feasibility studies
Capacity studies, process sizing
Preliminary design
Building permit
Project management
Construction supervision
Commissioning assistance
QC / IQ / OQ, PQ qualifications
Ergonomics & Workstation optimisation
Through its business divisions, ATRIX offers a comprehensive range of consulting and expertise services tailored to the life sciences sector. These services are provided within the framework of industry-specific framework contracts or specific assignments, the conclusions of which provide the insights required to make the decisions that ATRIX Group's customers need to make.








